

The Latin ‘aurum’ is the word that inspired the symbol for gold in the periodic table of elements (Au), but what gave gold its name is the specific color of this precious metal. They were all driven to travel a long way and search for gold led by the dream of becoming rich overnight. After hearing the information on the serendipitous discovery, more than 300,000 people hurried to Sutter’s Mill in Coloma, California. This discovery triggered the “ Gold rush ”. As he tried to sweep away the drops of sweat from his forehead, he noticed a shiny nugget on the ground reflecting the sunlight. The scorching hot rays made James Marshal pause a bit from the work. Marshall was building a sawmill for John A. It happened in 1848, in California, United States when one James W. The modern-day story of how gold was discovered includes a truly fortunate stroke of serendipity. It’s the earliest metal used by the Egyptians, who considered this chemical substance as a symbol of affluence and beauty. The ancient civilizations were familiar with gold. Due to this property, gold can be refined and detected in metal-made objects using the nitric acid test. The nitric acid contained in this chemical mixture can dissolve silver by itself, but this is not the case with gold. This soft and malleable member of the transition metals family of elements in the periodic table has an electronegativity of 2.54 according to Pauling, whereas the atomic radius according to van der Waals is 166 pm.ĭespite being resistant to most of the acids, gold can dissolve in nitric acid and hydrochloric acid mixture labeled as aqua regia. With the periodic table symbol Au, atomic number 79, the atomic mass of 196.967 g.mol -1, and electronic configuration 4f145d106s1, gold is a ductile metal that reaches its boiling point at 2836☌, 5137☏, 3109 K, while the melting point is achieved at 1064.18☌, 1947.52☏, 1337.33 K. The energy of the third ionization: X 2+ → X 3+ + e −ĭiscovery date: In 1848 by James W. The energy of the second ionization: X + → X 2+ + e − The energy of the first ionization: 9.2257 eV The symbol in the periodic table of elements: AuĮlectronegativity according to Pauling: 2.54 Fact Box Chemical and Physical Properties of Gold Being a member of the transition metals family of periodic table elements, gold has one valence electron that supports the formation of univalence compounds with this chemical element.
GOLD ELEMENT ATOMIC NUMBER FREE
It’s a precious metal found in its free elemental form in the beds of streams, alluvial deposits, and rock veins.
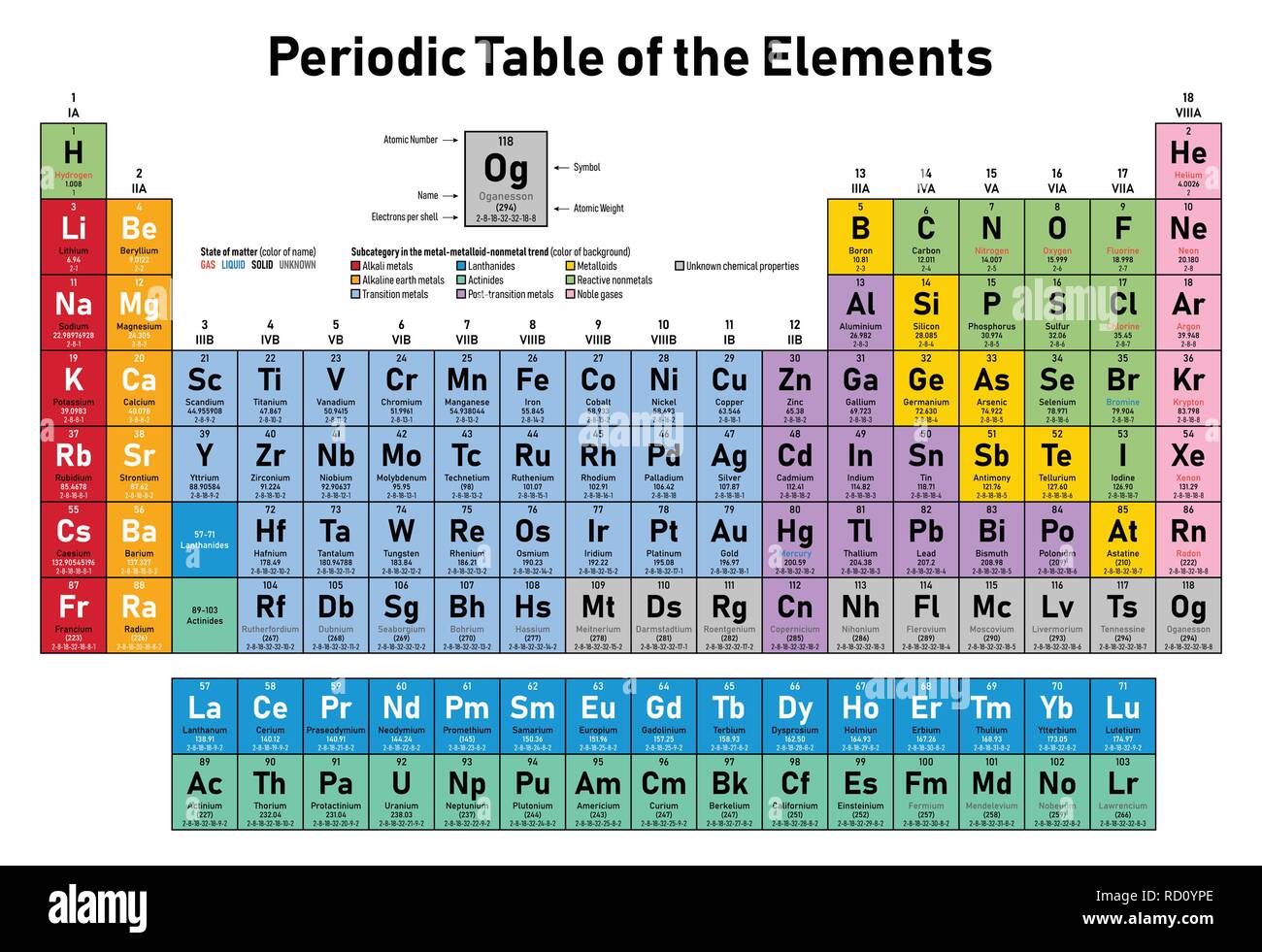
You will need to refer to a periodic table for proton values.Gold is a chemical element with an atomic number of 79 in the periodic table of elements. In this notation, the atomic number is not included.
Symbol-mass format for the above atom would be written as Cr-52. For an example of this notation, look to the chromium atom shown below:Īnother way to refer to a specific atom is to write the mass number of the atom after the name, separated by a hyphen. The "A" value is written as a superscript while the "Z" value is written as a subscript. Both the atomic number and mass are written to the left of the chemical symbol.

The composition of any atom can be illustrated with a shorthand notation called A/Z format.


 0 kommentar(er)
0 kommentar(er)
